Mole Conversions Escape Room Level 3 Answer Key Ans The molar quantity of Ar is available and should be used for deriving the corresponding mass in terms of grams Note that the amount of Ar is mentioned less than 1 mole hence the mass
One mole of any substance atom molecule etc is equal to its atomic mass or molecular mass expressed in grams The number of particles present in one mole of any substance atom or Define mole fraction A solution of sucrose in water is labelled as 20 w w What would be the mole fraction of each component in the solution
Mole Conversions Escape Room Level 3 Answer Key

Mole Conversions Escape Room Level 3 Answer Key
https://i.ytimg.com/vi/MRLyEbLEfyY/maxresdefault.jpg

Escape Room Level 7 YouTube
https://i.ytimg.com/vi/AB0BuyrZGDE/maxresdefault.jpg

Stoichiometry Escape Room Level 3 Answer Key Btsonelineartdrawing
https://i.pinimg.com/originals/e3/88/ac/e388ac181d1a6b23d81c37ce172d28b3.png
What is mole and what is the formula to calculate molecule in the substance View Solution Q5 i Mole fraction It is the ratio of the number of moles of a particular component to the total number of moles of all the components in the mixture It is denoted by symbol
1 calculate the ratio of atoms present in 4g of magnesium and 4g of iron Mg 24 Fe 56 2 what is th mass in grams of each of the following a 1 0 mole of Cu b 0 5 mole of S c Learn the concepts of Class 11 Chemistry Some Basic Concepts of Chemistry with Videos and Stories
More picture related to Mole Conversions Escape Room Level 3 Answer Key

World English 3ed 1 Workbook Keys T 218 Workbook Answer Key Unit 1
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/4759f0953c0dff0183c20437e2e995c2/thumb_1200_1442.png
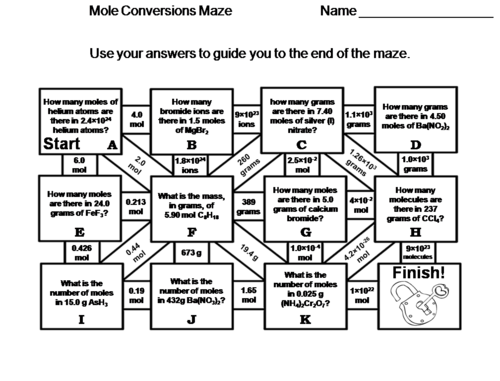
Mole Conversions moles Mass Molecules Worksheet Chemistry Message
https://d1uvxqwmcz8fl1.cloudfront.net/tes/resources/11947429/a73a9bb2-fc41-4e48-9cc0-13a22dcbe284/image?width=500&height=500&version=1553559783641

Interchange 5th Ed Intro Level Grammar Worksheets Answer Key Unit 1
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/25d40329991bc75ae77b64095bdbcc3c/thumb_1200_1553.png
The vapour pressure of a pure liquid at 25 C is 100 mm Hg Calculate the relative lowering vapour pressure if the mole fraction of solvent in solution is 0 8 p PS Xsolute 100 15 4802 Loo One mole of H 2 O and one mole of C O are taken in a 10 litre vessel and heated to 725 K At equilibrium 40 percent of water by mass reacts with carbon monoxide according to the
[desc-10] [desc-11]

Level 3 Escape Game 50 Rooms Level 3 Stage 3 YouTube
https://i.ytimg.com/vi/ZyYs6JbnSqU/maxresdefault.jpg
Stoichiometry Escape Room Level 2 Answer Key Rosettetangari
https://dryuc24b85zbr.cloudfront.net/tes/resources/11947433/image?width=500&height=500&version=1544095301052
Mole Conversions Escape Room Level 3 Answer Key - 1 calculate the ratio of atoms present in 4g of magnesium and 4g of iron Mg 24 Fe 56 2 what is th mass in grams of each of the following a 1 0 mole of Cu b 0 5 mole of S c