Labster Ionic And Covalent Bonds Walkthrough Labster Ionic and Covalent Bonding What information will help you chemically analyze the two mysterious substances Click the card to flip Click the card to flip andreaelaezm Reaction Kinetics The Essentials Thanos Car2 Preview Chemical Nomenclature ZyraSoto Preview Psychoactive drugs L3 Angelina Papakosta Chemistry Final Review kvannatta9
Ionic and Covalent Bonds Virtual Lab Labster 7 61K subscribers Subscribe Subscribed L i k e Share 2 2K views 1 year ago Join your friend on a quest to analyze two mysterious substances he 1 Use Interactive Models Core chemistry topics like the concept of interatomic bonds feel abstract as students cannot see atoms and electrons in real The simplified diagrams in their textbooks often fail to adequately explain the intricacies of bond formation This is where interactive models play a crucial role in teaching chemistry
Labster Ionic And Covalent Bonds Walkthrough

Labster Ionic And Covalent Bonds Walkthrough
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/1c436bb3118be18828516e65b1142c8d/thumb_1200_1553.png

Labster Ionic And Covalent Bonds Answers Exam Academy
https://tomdunnacademy.org/pictures/picture_1469.jpg

How Do Ions Increase Conductivity Atlas Scientific
https://atlas-scientific.com/files/ionic-covalent-bonds-diagram-1024x683.jpg
Following is a list of the relevant theory pages Periodic Table Atomic number Ions Anion Cation cat ion Ionic charge Chemical bonds Valence Electron Octet Rule Ionic compounds Ionic bonding Ionic solubility Ionic conductivity Crystal lattice Covalent compounds Covalent bonding Caffeine Glucose Lewis dot structures 1 Chemical Bonding is Abstract Chemical Bonding feels like an abstract concept The sub atomic particles called electrons within the microscopic atoms participate in chemical bonding It is impossible to visually imagine this type of reaction And without any attractive visuals the subject matter seems hard and dry for students 2
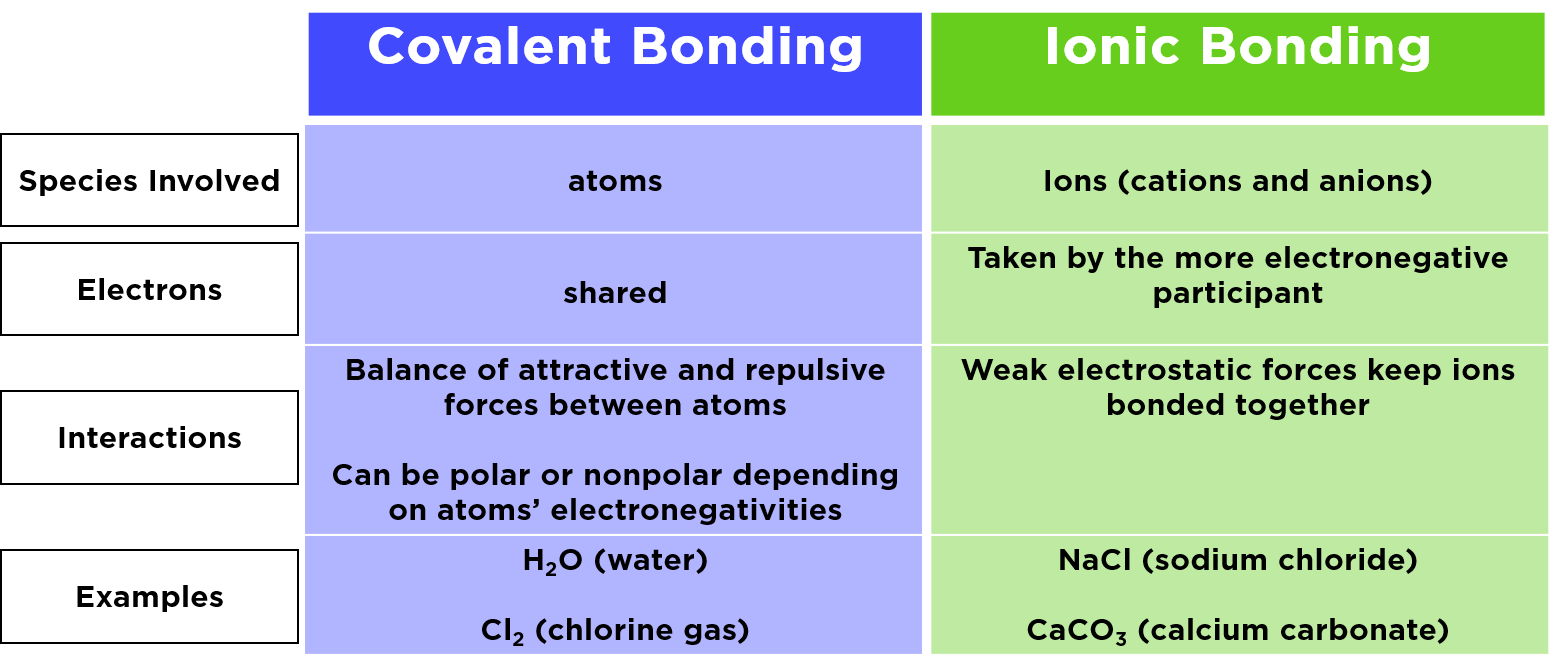
Ionic bonding is the complete transfer of valence electron s between atoms This type of chemical bond generates two oppositely charged ions There are many types of chemical bonds and forces that bind molecules together The two most basic types of bonds are characterized as either ionic or covalent In ionic bonding atoms transfer electrons to each other Ionic bonds require at least one electron donor and one electron acceptor In contrast atoms with the same electronegativity
More picture related to Labster Ionic And Covalent Bonds Walkthrough

Labs Notes With Dr White Lab Notebook Ionic And Covalent Bonds Sections Describe The
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/b1f2e5aebe74581e21a73a4cddd55654/thumb_1200_1553.png

Ionic Bond Definition Types Properties Examples
https://d2cyt36b7wnvt9.cloudfront.net/exams/wp-content/uploads/2021/06/08183431/1613-2048x1830.png
Ionic Bond Formation Compounds Expii
https://d20khd7ddkh5ls.cloudfront.net/covalent_and_ionic_bonds_table_2.png
Ionic bonds are formed between non metals and metals through an electron transfer The resulting ions are held together by the electrical attraction of opposite charges as for example in sodium chloride NaCl or potassium chloride KCl Figure 1 Ionic bonding leads to the formation of an ionic or crystal lattice When a cation cat ion forms an ionic bond with an anion an i on the number Check us out at https www inspirit academy Create your own ionic and covalent bo
Article Covalent Compounds Article Lewis Dot Structures Article Intermolecular forces Created by Labster Have you ever wondered how atoms are held together In this simulation you will learn the basics about atomic bonding in ionic and covalent compounds and h
Hybridization Labster
https://images.ctfassets.net/z51wgr36x3py/Sqi2YFYDSRGCBVGol8PsL/e43fd8b321d83ab6a3bcb29817ce546f/COC_5.PNG

Simulation 2 1 Ionic And Covalent Bonds This Simulation s Initial Goal Is To Teach Us The
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/b0fc93bc6d48864f5d055f798511a860/thumb_1200_1553.png
Labster Ionic And Covalent Bonds Walkthrough - Bonding Chemical bonding refers to any of the forces that hold atoms together in making compounds or molecules Chemical bonds include covalent ionic metallic and hydrogen bonding Related simulation Chemistry Ionic and Covalent Bonds
