Boiling Point Of Water At 2 Atm Online calculator figures and tables showing boiling points of water at pressures ranging from 14 7 to 3200 psia 1 to 220 bara Temperature given as C F K and R
This boiling point calculator tells you how to calculate the boiling point of most common substances at an arbitrary pressure based on the Clausius Clapeyron relation Whether you want to analyze water ethanol or ammonia simply provide some reference values and this calculator will do the work for you Water boiling point in pressure higher than atm The boiling point of water at atmospheric pressure 101315 Pa is 100c however the boiling point is changing with pressure this page is giving tables and abacus to know what is the boiling point of water at different pressures 1 Water boiling point at vacuum pressure
Boiling Point Of Water At 2 Atm

Boiling Point Of Water At 2 Atm
http://docs.engineeringtoolbox.com/documents/926/water-pressure-boiling-temperature-2.png

The Boiling Point Of Water At 1 Atm Is 100 C And The Boiling Point Of
https://img.homeworklib.com/questions/375693c0-6fe2-11ea-a8b8-8f1fb729844a.png?x-oss-process=image/resize,w_560

Phase Change Diagram Of Water Overview Importance Expii
https://d20khd7ddkh5ls.cloudfront.net/img_dff5d8f31ef7-1.jpeg
Reference tables contain values of the boiling point of water at different pressures in different unit of measure Legend P pressure mbar bar torr art or atom t temperature C The boiling point of water at a pressure in mbar For example for water the boiling point is 100 C at a pressure of 1 atm The boiling point of a liquid depends on temperature atmospheric pressure and the vapor pressure of the liquid When the atmospheric pressure is equal to the vapor pressure of the liquid boiling will begin
The boiling point of water is the temperature at which the vapor pressure of water equals the pressure surrounding the water and bubbles of vapor form inside the liquid At standard atmospheric pressure 1 atm or If you want a quick and simple answer you can say that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure sea level However the value is not a constant
More picture related to Boiling Point Of Water At 2 Atm
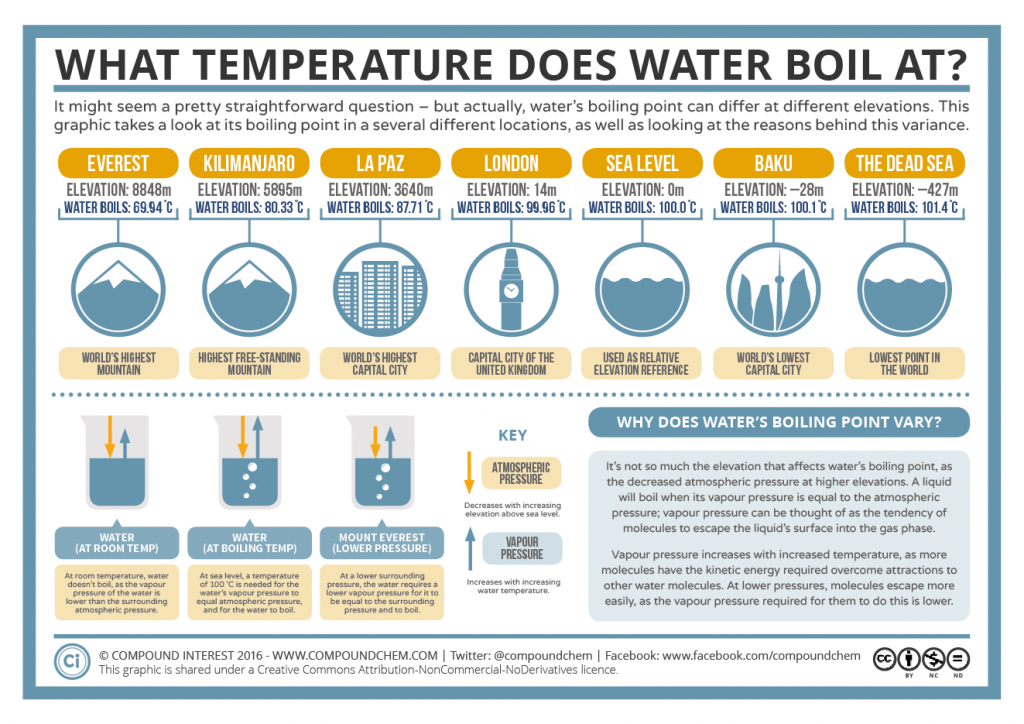
What Temperature Does Water Boil At Boiling Point Elevation
http://www.compoundchem.com/wp-content/uploads/2016/03/The-Boiling-Point-of-Water-at-Different-Elevations-1024x724.png
Thermal Secrets To Boiling Point Calibration
https://blog.thermoworks.com/wp-content/uploads/2014/11/BoilingThermapen-1.png

Boiling Point Of Water What Temperature Does Water Boil
https://sciencenotes.org/wp-content/uploads/2020/08/Boiling-Point-of-Water.png
If this pressure is the standard pressure of 1 atm 101 3 kPa then the temperature at which the liquid boils is referred to as its normal boiling point This is the boiling point which is usually quoted in chemical literature The boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the water and the water changes into a vapor Water at high pressure has a higher boiling point than when that water is at atmospheric pressure
[desc-10] [desc-11]
Boiling Point Of Water At Sea Level In Kelvin
https://lh6.googleusercontent.com/proxy/S18hFsonItHqscDhAFPDXr9Kz6a7gXJd-wzQMw9lgE7D4kM0-UC_vrEQ6UId7lYKTyXZFaBHwC2E_0z7j5uqf2cE8gVR5IETDUiV3duhZo01=s0-d

Saturation Temperature boiling Point KENKI DRYER
https://i1.wp.com/kenkidryer.com/wp-content/uploads/2020/06/differenc-in-saturated-temperature-boiling-point-by-pressure-圧力による飽和温度の違い-2020.6.18.png?ssl=1
Boiling Point Of Water At 2 Atm - Reference tables contain values of the boiling point of water at different pressures in different unit of measure Legend P pressure mbar bar torr art or atom t temperature C The boiling point of water at a pressure in mbar